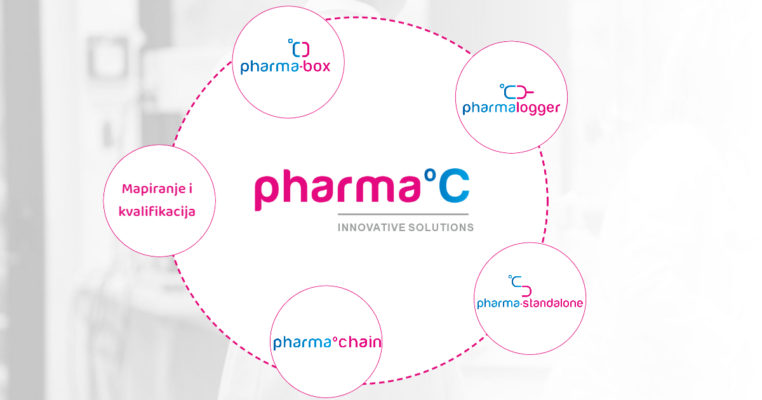
GARANCIJA KVALITETE HLADNOG LANCA
Standard predstavlja potvrdu kupcu da ljekarna koja ga posjeduje vrši nadzor i brine o ispravnosti lijekova koje pacijent konzumira. Kako bi lijek stigao ispravan do pacijenta potreban je stalni nadzor temperature i pravovremena reakcija u slučaju temperaturnih odstupanja. Standard je kreiran za proizvođače lijekova, veledrogerije i ljekarne.
Farmaceutski i medicinski hladnjaci
Lijekovi koji zahtijevaju uvjete hladnog lanca spadaju među najosjetljivije, a samim time i u najkritičniju skupinu lijekova. Zbog toga farmaceutske i medicinske hladnjake možemo smatrati kritičnom i zahtjevnom opremom koju treba pomno odabrati
Ponuda farmaceutskih i medicinskih hladnjaka
Nadzor temperature u prostoru ljekarne
Brzo i jednostavno proširenje postojećeg Pharmalogger sustava u prostorima ljekarne.
Kontaktiraj nas


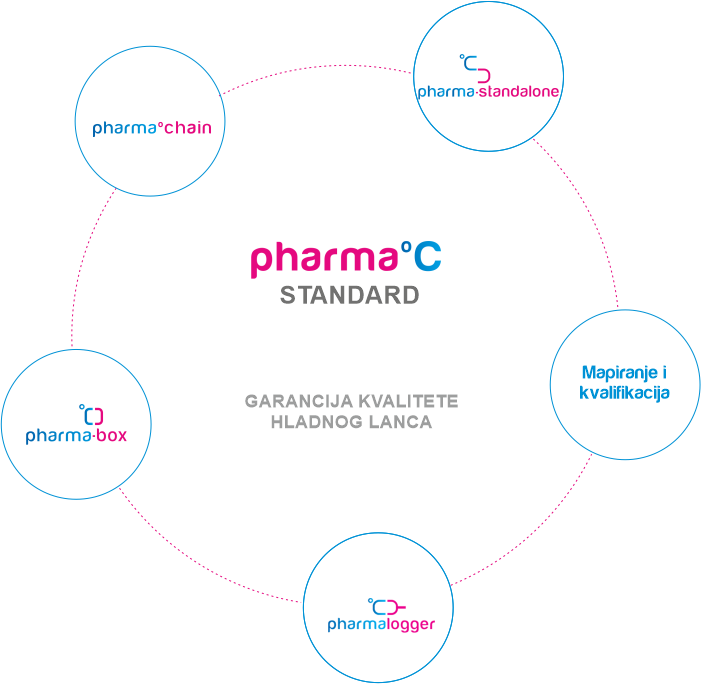
GARANCIJA KVALITETE HLADNOG LANCA
Standard predstavlja potvrdu kupcu da ljekarna koja ga posjeduje vrši nadzor i brine o ispravnosti lijekova koje pacijent konzumira. Kako bi lijek stigao ispravan do pacijenta potreban je stalni nadzor temperature i pravovremena reakcija u slučaju temperaturnih odstupanja. Standard je kreiran za proizvođače lijekova, veledrogerije i ljekarne.

Farmaceutski hladnjaci
Lijekovi koji zahtijevaju uvjete hladnog lanca spadaju među najosjetljivije, a samim time i u najkritičniju skupinu lijekova. Zbog toga farmaceutske hladnjake možemo smatrati kritičnom i zahtjevnom opremom koju treba pomno odabrati
Ponuda farmaceutskih hladnjaka
Nadzor temperature u prostoru ljekarne
rzo i jednostavno proširenje postojećeg Pharmalogger sustava u prostorima ljekarne.
Kontaktiraj nas



GARANCIJA KVALITETE HLADNOG LANCA
Standard predstavlja potvrdu kupcu da ljekarna koja ga posjeduje vrši nadzor i brine o ispravnosti lijekova koje pacijent konzumira. Kako bi lijek stigao ispravan do pacijenta potreban je stalni nadzor temperature i pravovremena reakcija u slučaju temperaturnih odstupanja. Standard je kreiran za proizvođače lijekova, veledrogerije i ljekarne.

Farmaceutski hladnjaci
Lijekovi koji zahtijevaju uvjete hladnog lanca spadaju među najosjetljivije, a samim time i u najkritičniju skupinu lijekova. Zbog toga farmaceutske hladnjake možemo smatrati kritičnom i zahtjevnom opremom koju treba pomno odabrati
Ponuda farmaceutskih hladnjaka

Nadzor temperature u prostoru ljekarne
Brzo i jednostavno proširenje postojećeg Pharmalogger sustava u prostorima ljekarne.
Kontaktiraj nas


PHARMA°CHAIN
Farmaceutski i medicinski hladnjaci
Farmaceutski i medicinski hladnjaci za sigurno čuvanje lijekova. Kvalificirani prema GDP smjernicama i prilagođeni stvarnim potrebama ljekarnika.


PHARMA°CHAIN
Farmaceutski hladnjaci
Farmaceutski hladnjaci za sigurno čuvanje lijekova. Kvalificirani prema GDP smjernicama i prilagođeni stvarnim potrebama ljekarnika.




PHARMA – BOX
Što je Pharma – box?
Pametni spremnik za prijenos
termo-osjetljivih lijekova



PHARMALOGGER
Pharmalogger sustav
Automatizirani sustav za precizno mjerenje te nadzor temperature i relativne vlage u farmaceutskoj industriji
PHARMALOGGER
Pharmalogger sustav
Automatizirani sustav za precizno mjerenje te nadzor temperature i relativne vlage u farmaceutskoj industriji
PHARMALOGGER
Pharmalogger sustav
Automatizirani sustav za precizno mjerenje te nadzor temperature i relativne vlage u farmaceutskoj industriji
Saznaj višeO NAMA
Tko smo?
Vodeći proizvođač mjernih uređaja i sustava nadzora uvjeta okoline na hrvatskom tržištu i susjednim regijama
Pružamo usluge akreditiranih umjeravanja (ISO 17025) te kvalifikacije prostora i hladnog lanca prema GxP smjernicama
Stvaramo rješenja za adekvatno čuvanje lijekova i ostalih termo-osjetljivih proizvoda u svakom trenutku skladištenja i distribucije
O NAMA
Tko smo?
Vodeći proizvođač mjernih uređaja i sustava nadzora uvjeta okoline na hrvatskom tržištu i susjednim regijama
Pružamo usluge akreditiranih umjeravanja (ISO 17025) te kvalifikacije prostora i hladnog lanca prema GxP smjernicama
Stvaramo rješenja za adekvatno čuvanje lijekova i ostalih termo-osjetljivih proizvoda u svakom trenutku skladištenja i distribucije
O NAMA
Tko smo?
Vodeći proizvođač mjernih uređaja i sustava nadzora uvjeta okoline na hrvatskom tržištu i susjednim regijama
Pružamo usluge akreditiranih umjeravanja (ISO 17025) te kvalifikacije prostora i hladnog lanca prema GxP smjernicama
Stvaramo rješenja za adekvatno čuvanje lijekova i ostalih termo-osjetljivih proizvoda u svakom trenutku skladištenja i distribucije
Novosti

Novosti

Novosti
Preko 600 zadovoljnih korisnika





Preko 600 zadovoljnih korisnika





Preko 600 zadovoljnih korisnika